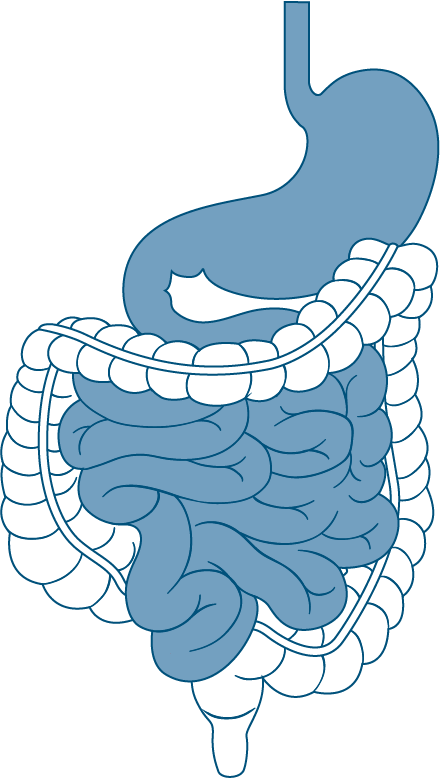
Upper GI tract
Dynamic model of digestion simulating the stomach and small intestine of the gastrointestinal tract
The first compartments of the SHIME® platform sequentially simulate the stomach, duodenum, jejunum, and ileum, reproducing the spatiotemporal changes of physiological parameters (pH, salt concentrations, residence times, in vivo correlated volumes, enzyme concentrations, bile salt concentrations,…) of the upper gastrointestinal tract, incorporating the parameters defined in the INFOGEST 2.0 method. The simulation can be performed under fasted or fed conditions (in the absence or presence of food, respectively) as it would occur in vivo. This system offers a comprehensive upper GI simulation with a focus on the stomach biome and stomach microbiome, ensuring the simulation mirrors real-life conditions in the digestive system.
Personalized in vitro simulations adapted to the physiology of various hosts
By adapting the parameters, the Upper GI tract simulator can be adjusted to simulate specific population groups like babies, children, and the elderly, specific disease conditions (e.g., pancreatic enzyme deficiency, specific diets, and even other species (e.g., cat, dog, and pig). When coupled to a short-term colonic simulation, the entire gastrointestinal transit in a function of time can be simulated. The upper GI model can be fine-tuned to accommodate variations in digestion processes due to host-specific factors, including microbiome differences.
Bioaccessibility, bioavailability, digestion, absorption, capsule and tablet dissolution, stability, probiotic survival,…
What do you want to know?
The upper GI tract simulation is a reliable, reproducible, and validated technology to assess the behaviour and fate of virtually any ingestible test product. This technology can be used to reliably predict the dissolution of capsules and tablets; the release profile of actives from formulations, including targeted delivery or delayed release strategies; drugs and products stability and protection from the harsh environment of the gut; and the survival and metabolic activity of live biotherapeutics and probiotics. By including a dialysis membrane, the kinetics of digestion of food products, carbohydrates and proteins, and the bioaccessibility and bioavailability (absorption) of nutraceuticals, minerals, vitamins, and toxins can be studied in detail. Likewise, inclusion of epithelial cell lines allows studying active transport and absorption of actives, nutraceuticals, vitamins, metabolites, and drugs from the small intestine. This gastric and intestinal simulation enables a clear understanding of how nutraceuticals and pharmaceuticals behave in the digestive tract, improving formulation design and product optimization. The use of an in vitro stomach model enhances the study of digestion and absorption processes, making it a powerful tool for research into digestion absorption models.
The detailed insight on the behaviour of test products using Upper GI tract simulations will allow ranking and improvement of products and their formulation in a cost-effective and time-efficient manner. The upper gastrointestinal simulator provides crucial data that can significantly reduce time in preclinical and clinical studies, making it an indispensable tool for advancing product development in the health and wellness industry.
Research endpoints
Survival of probiotics and LBPs
- Plate count
- PMA-qPCR
- Flow cytometry
Digestion and absorption
- Mineral analysis
- Carbohydrate profiling (HPAEC-PAD)
- Kjeldahl & AA analysis
- UHPLC
- LC-HRMS
- …
Capsule degradation and release of its content
Project-specific detections and analyses
Investigational topics