Recent Posts:

Written By
Sharmain Zain
(Marketing Lead)
Making Better Gut Health Decisions With IVIVC Validated Model
You can get data from almost any gut model. But the truth behind the gut? That’s harder to find.
Gut research doesn’t suffer from a lack of models — it suffers from a lack of relevance.
You can simulate fermentation. You can measure shifts in microbial composition. But unless your model reflects what actually happens in the human body, the data won’t take you far.
That’s why trusting early-stage results depends on one thing: whether the model behind them was built to reflect the real world. But how do you know if a model is truly reliable?
Not because it looks complex. Not because it runs smoothly. And definitely not because it produces neat graphs.
The real test is whether its results hold up — not just under ideal lab conditions, but when compared to actual human outcomes. That’s where most models fall short. And where validation becomes non-negotiable.
Because in gut research, relevance isn’t optional — it’s what gives data meaning.
That’s what in vitro–in vivo correlation (IVIVC) offers: not just a technical milestone. It’s a marker of credibility — proof that what happens in a lab can predict what happens in real life.
Colon-on-a-plate®: Fast Doesn’t Have to Mean Shallow
High-throughput gut models are often built for speed — but speed alone doesn’t make them useful. Without depth, fast results just get you to the wrong conclusion faster.
Colon-on-a-plate® was developed to close that gap. It’s a short-term, miniaturized colonic model that allows rapid testing of multiple donors, conditions and test products — but with the same demand for validation that applies to any serious gut model.
Its IVIVC isn’t theoretical. It’s been demonstrated through studies showing strong alignment between ex vivo fermentation results and in vivo outcomes — from SCFA production and microbiota shifts to immune modulation and barrier integrity.
That means researchers aren’t just screening faster — they’re working with a system that gives them real confidence in what comes next.
Case in Point: NUTRIOSE®
The predictive strength of Colon-on-a-plate® becomes clear when compared directly to in vivo outcomes — as shown in a study using NUTRIOSE®, a resistant dextrin with known prebiotic effects.
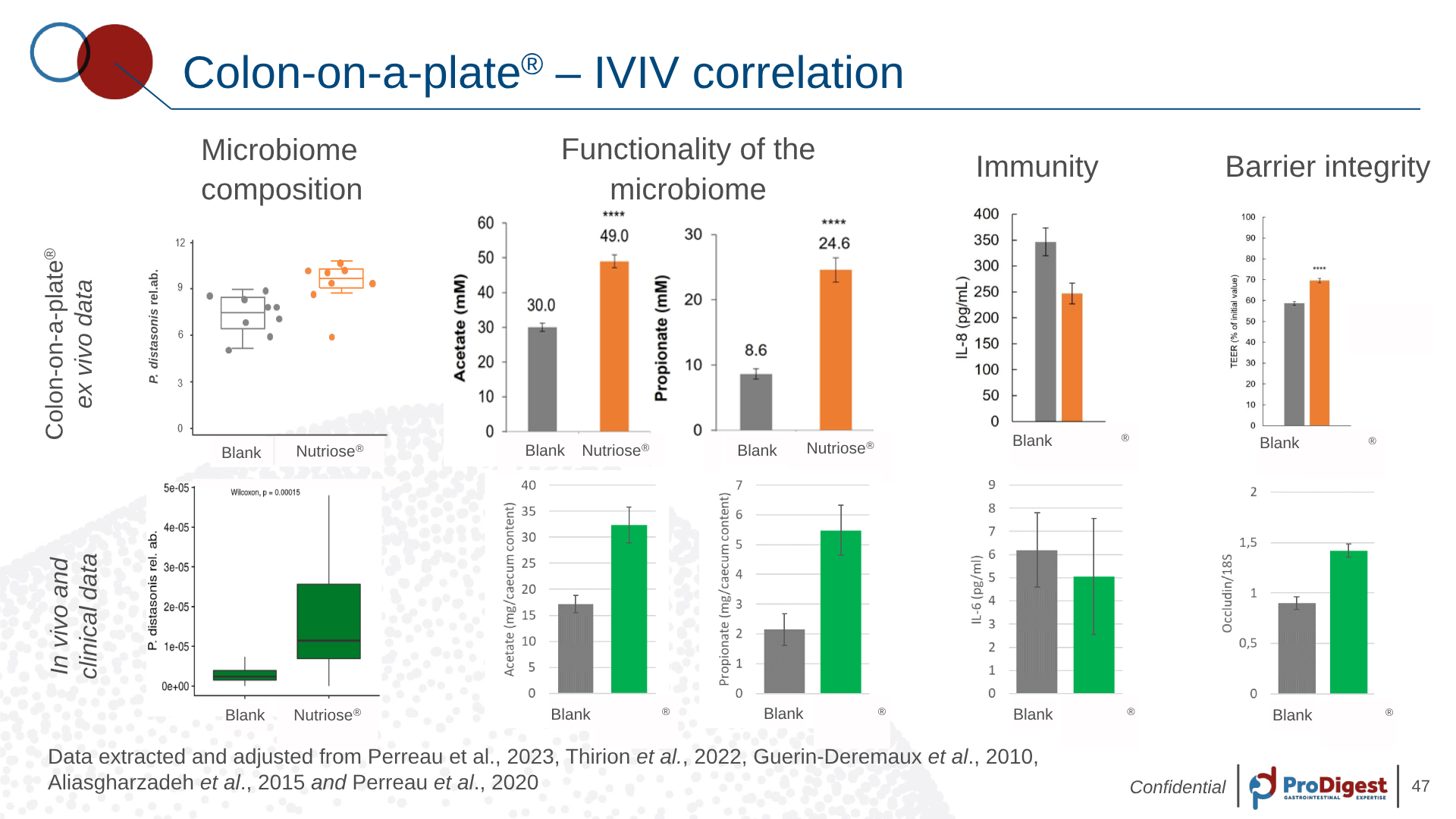
After 48 hours of fermentation with stool from eight healthy donors, the model revealed:
- A consistent increase in Parabacteroides distasonis
- Significantly higher levels of acetate and propionate
- Improved gut barrier function (measured by TEER and occludin expression)
These ex vivo findings didn’t stand alone. When compared to in vivo and clinical datasets, the results aligned — across microbial composition, metabolite output, and host response.
This is IVIVC in practice: a compound tested in a high-throughput lab setting, showing outcomes that are not only measurable — but meaningful.
Colon-on-a-plate® offers a fast, controlled view into how microbial communities respond to a compound. It’s ideal for capturing early functional signals — shifts in composition, metabolite output, or immune interaction that may carry through in vivo.
But early signals aren’t always the full story.
SHIME®: The Whole Gut, Not Just a Snapshot
Some studies demand more: insight into how a compound moves through the entire gastrointestinal tract, how it behaves under repeated dosing, and how its effects evolve over time — not just in hours, but over days or weeks.
That’s where SHIME® comes in — a dynamic, full-gut model that replicates the entire digestive journey. From stomach to colon, from single ingredients to complex formulations, SHIME® helps researchers track what’s happening, when it happens, and why it matters. Because some research questions take time to answer — and SHIME® is built to keep up.
It’s one of the most validated and representative ex vivo models available today, designed to simulate the physiological, chemical, and microbial conditions of the entire gastrointestinal tract. From stomach to colon, SHIME® tracks how compounds are digested, fermented, and metabolically transformed — and how those changes affect the microbiome over time.
The value isn’t just in what it simulates. It’s in what’s been proven.
With over 150 peer-reviewed publications, SHIME® is one of the few models with a demonstrated track record of alignment with in vivo results. It’s been used across sectors — from prebiotics, probiotics, and synbiotics to dietary fibers, postbiotics, and therapeutic compounds — to generate data that actually translates.
Running up to three weeks, SHIME® makes it possible to monitor long-term effects: microbial adaptation, metabolite production, barrier modulation, and immune-related changes. And because it preserves the original structure of the donor microbiome — shaped by diet, medication, and lifestyle — it provides insight into how real-world variation and interindividual variation influences product performance.
Whether you’re validating a formulation or de-risking a clinical trial, SHIME® offers a flexible, mechanistic platform to ask better questions — and trust the answers.
Case in Point: Hops Beer
Hops — the plant behind beer’s bitterness — contain prenylflavonoids, including 8-prenylnaringenin (8-PN), one of the most potent phytoestrogens found in nature. Because 8-PN levels in beer are low, its health impact from moderate consumption was long considered negligible.
But the gut microbiome changes that picture.
Human intestinal bacteria can convert isoxanthohumol (IX) — another hop-derived compound — into 8-PN. In some individuals, this transformation can increase 8-PN exposure up to 10-fold.
Using the SHIME® model, researchers followed the full digestive journey of these compounds. IX and other prenylflavonoids passed unchanged through the stomach and small intestine. The critical transformation happened only in the distal colon, where up to 80% of IX was converted to 8-PN.
To assess individual variability, IX was incubated with fecal samples from 51 women. Based on 8-PN production levels, microbiota profiles were grouped into:
- High producers (8)
- Moderate producers (11)
- Slow producers (32)
Three participants — one from each group — were then given 5.59 mg of IX daily for four days. Their urinary 8-PN excretion matched the predicted microbial activity from SHIME®, showing a strong correlation (R² = 0.6417, P < 0.01).
This study shows that even moderate beer consumption can result in significant 8-PN exposure — but only in people whose microbiota can make the conversion.
And SHIME® helped pinpoint exactly where, when, and in whom that happens.
Long-term models like SHIME® give you the full picture. But not every research question calls for full-system depth. Sometimes, you’re not trying to map every mechanism — you’re trying to find out what works, fast.
That’s exactly where Screening SHIME® comes in.
Built on the same scientific foundations as SHIME®, this platform uses a single colonic reactor that reflects the full colonic microbiome — without splitting into proximal and distal regions. That simplification isn’t a compromise. It’s what makes Screening SHIME® the fastest, highest-throughput platform in the SHIME® family.
The setup supports parallel testing across multiple donors and conditions, enabling teams to screen dietary compounds, formulations, or microbial strains in real-world-like environments — all in just 8 days.
With automated control, repeat dosing, and optional offline extension with co-culture models (like Caco-2 / THP-1), Screening SHIME® offers more than just a fast answer. It gives you early insights into microbial shifts, metabolite profiles, and even functional host interactions — without the time or cost of a full-scale model.
It’s the ideal tool when you’re comparing what’s worth pursuing — and need to make those calls before committing to deeper investigation.
In short: faster decisions, same scientific standard.
These Models Set the Standard — But They’re Not the End
Colon-on-a-plate®. SHIME®. Screening SHIME®.
Each was built for a different type of question. Each has been tested, refined, and validated — not just in theory, but in practice. Together, they’ve helped researchers generate insights that actually translate.
That foundation matters. Because it’s what we’re building on.
At Vitafoods Europe, we’ll be unveiling what comes next — a new evolution in ex vivo gut research, shaped by everything these models have made possible.
More flexibility. More speed. Same scientific standard.
Stay tuned.
Recent Posts:

Written By
Sharmain Zain
(Marketing Lead)